Last Monday, 5 May 2025, the ECOBAT team from KU Leuven took part in the annual SIM² KU Leuven Researchers Meeting, an event that gathered the scientific community of the SIM² institute. Held on KU Leuven campus, the event brought together professors, postdoctoral researchers, PhD candidates, and master’s students (over 80 members out of 386 total members) for a day of knowledge exchange and networking.
SIM² KU Leuven is one of the university’s flagship interdisciplinary research institutes. Its mission is to advance sustainable production and recycling of strategic and critical materials through cutting-edge science, education, and societal engagement. This year's theme focused on lithium, an element at the core of the energy transition. Lithium research at SIM² covers the entire lithium production value chain from mining and refining to battery production and recycling. ECOBAT, the KU Leuven research initiative on next-generation battery technologies, was well represented at the event.
Dr. Peter Tom Jones, SOLVOMET Valorization manager SIM² director, opened the meeting with a broad overview of SIM²'s latest developments. Following that, Nand Peeters and Liuba Lukina gave a talk showcasing how SIM² KU Leuven is pushing the boundaries of lithium research. They highlighted the efforts of 12 professors, ranging from geological exploration to the development of new battery chemistries and closed-loop recycling strategies.
Particular attention was given to battery innovations led by members of the Fransaer Research Group, part of the Department of Materials Engineering. Professor Jan Fransaer’s group focuses on fundamental and applied electrochemistry, with a team of five postdoctoral researchers and six PhD students. Their work includes recovering metals through electrochemical processes and developing both lithium and post-lithium battery technologies.
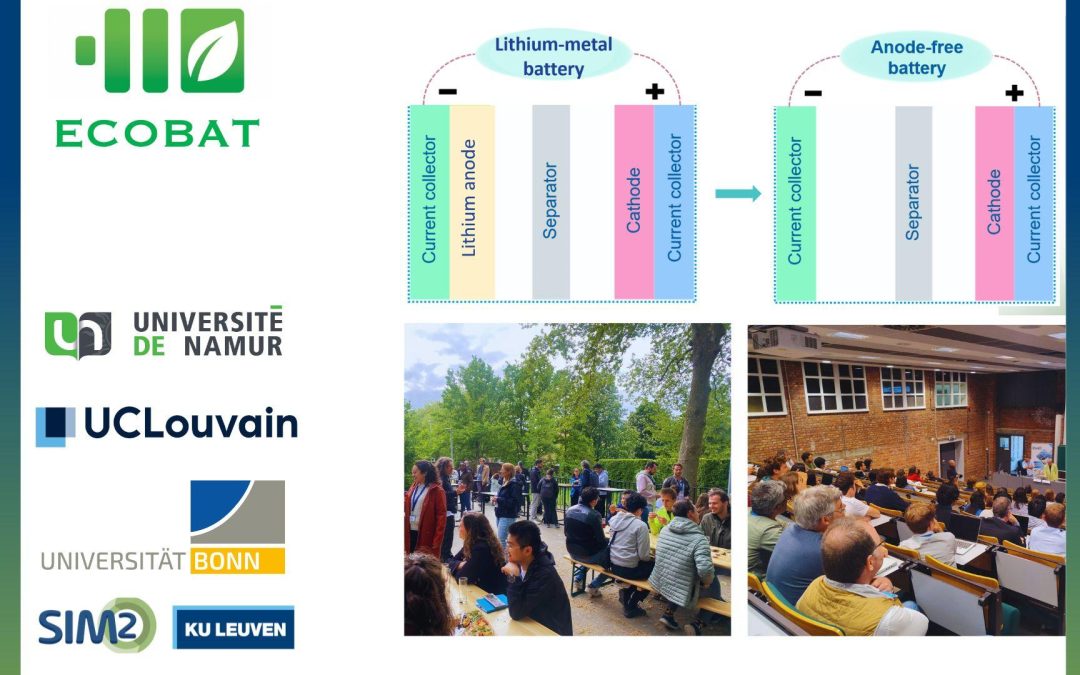
The group’s ECOBAT-linked research includes high-energy-density lithium metal and anode-free batteries. Unlike conventional lithium-ion batteries that use graphite anodes, lithium metal batteries use pure lithium metal, offering up to ten times the specific capacity. This dramatically increases the energy stored per unit mass and volume. Yet even beyond lithium metal batteries lies the holy grail: the anode-free lithium battery.
In anode-free batteries, there is no lithium metal at the anode side, just a simple copper foil or similar current collector. During charging, lithium ions from the electrolyte are plated onto the copper; during discharge, they are stripped away. This minimalist design improves energy density, reduces costs, and enhances safety. However, it introduces significant challenges, particularly related to the unstable deposition of lithium and parasitic reactions with the electrolyte.
To tackle this, ECOBAT researcher Zhenyu Zhou developed an innovative electrode design. By coating copper foil with a nanoparticle layer, he achieved smooth and uniform lithium deposition. This improvement significantly reduces dendrite formation and unwanted reactions, resulting in longer-lasting and higher-performing full-cell batteries.
The ECOBAT team's participation in the SIM² Researchers Day was a valuable way to showcase their scientific progress and forge new collaborations within the SIM² institute.